The Clinical Trials Toolkit
The Clinical Trials Toolkit provides practical advice to researchers in designing and conducting publicly funded clinical trials in the UK.
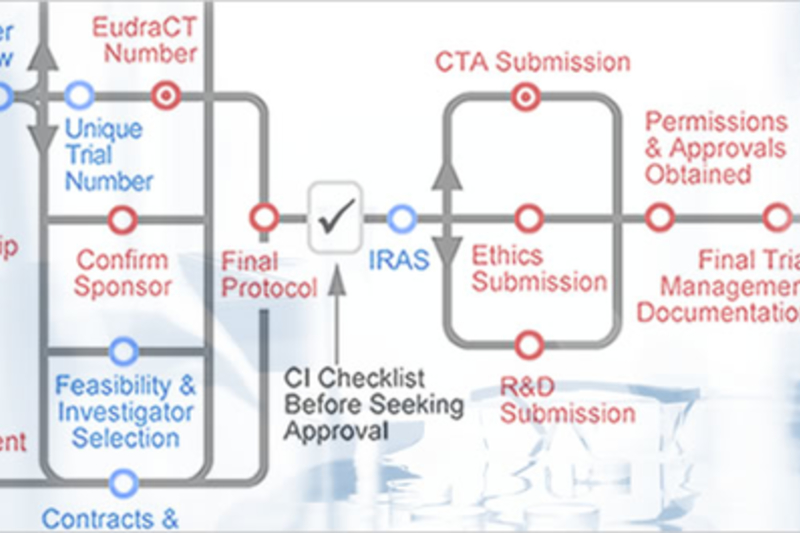
About the Toolkit
Through the use of an interactive routemap, this site provides information on best practice and outlines the current legal and practical requirements for conducting clinical trials.
The Toolkit is primarily focused on Clinical Trials of Investigational Medicinal Products (CTIMPs) and the regulatory environment and requirements associated with these. However researchers and R&D staff working on trials in other areas will also find useful information and guidance of relevance to the wider trials environment.
Recent changes to the Toolkit
September 2024 - The Clinical Trials Toolkit has now been refreshed.
June 2023 - Content updates to the Informed Consent and Dissemination of Results stations.
June 2023 - Content updates to the Informed Consent and Dissemination of Results stations.

